- Home
- Solutions
- Quality Processes
- Change Control
- Deviation
- Investigation
- CAPA Management Software
- Market Complaint
- Document Control
- SOP
- Protocol
- Work Instruction
- Specification
- Controlled Copy Release (Doc Issuance)
- Electronic Training Records
- eLearning
- Training Records Management
- Training Scheduling
- Product Quality Review (PQR)
- Vendor Quality
- Bundled Packages
- Resources
- About
- Contact us
Digitalization of 6M Investigation
Business Situation
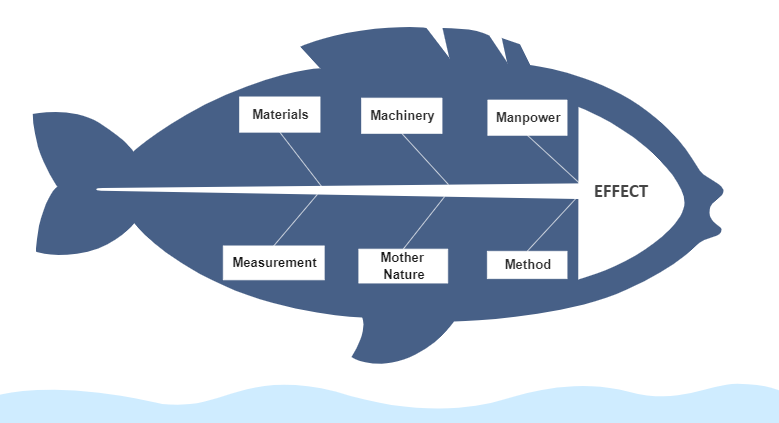
Investigations of production and industrial operations problems are a very integral part of any pharmaceutical or life science industry. Investigation of any kind of problem or issue, deviation, or failures in a process in an organization can be considered as a substructure that can bring increased efficacy to the system. Multiple tools are used in the pharmaceutical industries to solve complex problems related to areas of production, quality, or any system in general. 6M is one such tool, used extensively in Pharmaceutical or Production industries, that identifies root cause or probable root cause of any issue by exploring problems in multiple directions. It dissects the problems into 6 simple sections, i.e., Material, Method, Machine, Mother Nature, Measurement, and Man. Digitalization of 6M investigation is what organisations need to make this simple.
Although the simplicity and robustness of this tool is its USP, the manual preparation, maintenance, and storage the 6M investigation documents generally bring obscurity during the process of investigation. The confusion may increase in manual paper-based work environment scenarios as interlinked documents of the 6M investigation (for instance, CAPA) may be difficult to search as well as store, and make available at the time of need.
Business Complexity
Paper based work is error prone which can be a huge drawback in industries that hugely rely on accuracy. Organizations that are still in the paper-based work environment may deal with several complexities during investigation that can be solved with digitalization of 6M investigation:
- Lack of transparency in documenting the root cause
- Time consuming
- Lack of control and communication between investigating team
- Unable to track and record the status
- Improper intermediate storage of paper-based documents leads to misplacement of records
- Unavailability of interlinked documents due to poor storage management
- Inaccuracy of investigation due to discrepancy, leading to wrong or null identification of root cause
- Above factors may lead to delayed or incorrect decision making
Solution for Digitalization of 6M Investigation
Benefits Derived by Sarjen QEdge 6M Analysis Tool
- Provides an easy workflow & framework
- Facilitates easy flow of document
- Helps the entire investigation team to remain on the same page during the brainstorming, due to its 360⁰ accessibility
- Supports viewing of the entire investigation in single view unlike the multi-page paper-based 6M investigation documents, thus, allowing a deeper investigation
- Linked documents can be reviewed at just a press of a button
- Ultimately, reduces manpower and tiring manual work
- Cost Effective and Time saving
Start your digital journey today and meet our consultants to know how quality management for your clinical research organisations can be automated and made beneficial to you. Drop us an email at sarjen@sarjen.com or contact us to book a demo.
QEdge Quality Management Software Suite
Contact Us
6th Floor, Arista, 100 Feet Anand Nagar Rd, Jodhpur Village, Ahmedabad, Gujarat 380015
Phone : +91-79-66214899
Email: sarjen@sarjen.com

