- Home
- Solutions
- Quality Processes
- Change Control
- Deviation
- Investigation
- CAPA Management Software
- Market Complaint
- Document Control
- SOP
- Protocol
- Work Instruction
- Specification
- Controlled Copy Release (Doc Issuance)
- Electronic Training Records
- eLearning
- Training Records Management
- Training Scheduling
- Product Quality Review (PQR)
- Vendor Quality
- Bundled Packages
- Resources
- About
- Contact us
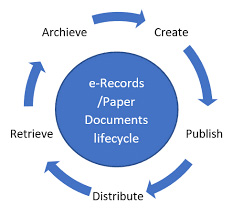
Important aspects of document distribution and retrieval in Document Automation Process
In regulatory businesses like Pharmaceutical, Healthcare and Lifesciences, document distribution plays a major role. Documentation is the most crucial activity in these businesses.
Preciously Documents such as Standard Operating Procedure, Testing Procedure, Protocol, Policies and many other regulated documents are easy to create. But, these are complex to manage along with its distribution and retrieval while managing manually.
Challenges of Paper based method
Broadly, paper-based document lifecycle mechanism is tedious, complex, improper and a panic job for employees. Employees must manage paper-based record according to lifecycle framework. If they are not equipped with such pragmatic mechanism they fail, and this results as finding during audit. Once findings appear on surface other trouble starts. Finally entire organization is held responsible in terms of responding and correcting those findings.
All regulated businesses know what standards to be followed in order to be compliant. Hence, they invest more time, extra money to remain compliant. Any company following manual systems may trap into noncompliance. Because of paper based manual mechanism which is a stringent process and there will be always be a risk of missing documents, version conflicts, control/uncontrol copy and much more.
For a company operating under strict regulation, management of paper-based issuance and retrieval activity invites its own problems. Lots of documents are managed through paper matrix, some on mail and few on soft copy. Further, proving lack of method to control the issuance. Original problem arises when it comes to retrieval. If it is not recorded, it becomes difficult to retrieve increasing the risk of audit failures.
How to leverage
An Electronic DocControl System or an Electronic Filing Cabinet in document distribution helps industry to digitize their documents in a secured, reliable and controlled manner. It starts from creation to archiving of the same document or we can term it as complete document lifecycle management.
Document Distribution
Document distribution is a key component of any DocControl system. It’s the way you can send documents to customers, vendors, and even internal employees including unique distribution code with the help of controlled workflow within the system with selection of appropriate watermark and audit trail.
Doc control system gives you the ability to distribute documents the way you need to, like download, print (Hardcopy/Softcopy) and email.
Document Retrieval
Document retrieval needs to be quick and easy after either fulfilling purpose of work or document is no longer effective for work. System gives the search features to be able to identify and retrieve a document on any keyword with the execution of controlled workflow.
for work. System gives the search features to be able to identify and retrieve a document on any keyword with the execution of controlled workflow.
DocControl system give you the ability to retrieve same distributed documents by identifying unique distribution code and you’ll be able to mark a comment which will reflect in audit trail.
Conclusion
It is not the end of any electronic Doc-Control system to mitigate the quality risk within the system. Today’s DocControl systems having plenty of key features which eases daily operation within enterprise. It can also talk with QMS and TMS gateway.
Done well, electronic distribution and retrieval gives more transparency during regulatory audit today, tomorrow and ten year down the road.
QEdge Quality Management Software Suite
Contact Us
6th Floor, Arista, 100 Feet Anand Nagar Rd, Jodhpur Village, Ahmedabad, Gujarat 380015
Phone : +91-79-66214899
Email: sarjen@sarjen.com