

Close

Where attendees learned about the best practices in the selection and implementation of Enterprise wide document control software from market, so for global businesses such as pharmaceuticals, clinical research organization and life science organization with multiple brands, and with revenues of over $10B each, they seek for regulatory compliance as a prerequisite to achieve business needs for all quality processes, Document control and training segment. This depicts a true representation of an excellent quality deployment across the organization.
Many organizations experience they have situations that include enormous systems with multiple plants using ambiguous electronic document resulting in a mixture of manual paper-based processes and spreadsheets in a clumsy and uneven manner. In addition to its being credibly inefficient, it also lacks in transparency and traceability. At the time of reporting, huge amounts of time were consumed on the manual and human intervention activities associated with record management. Hence we will focus on key points that supports the selection of the “best fit” document control software and delivered exceptional results upon deployment:
One core requirement for success with Doc Control team that is capable, passionate and versatile. Only if the team working on this is assigned from the beginning, they can understand each step while going live. The formation of the team should include regional and overseas business unit representatives and should be cross-functional with the appropriate subject matter expertise. A Doc Control will focus multiple prime of integration technology, many of which we learned can provide huge benefits for the deployment when done correctly. The team should include individuals with understanding of current or planned ERP, eQMS and LIMS platforms.
For easy going and achieving software project deployment milestone within appropriate timeline to generate requirement, involvement of and consultation of stakeholders from R&D, engineering, manufacturing, finance QA and QC provides the foundation for clear requirements avoiding the temptation to gather requirements too quickly from a limited group and perform a quick pass for validation is important to achieving buy-in when rolling out, but also provides a clear depiction of specific current pain points. Furthermore, this involvement will provide better vision when designing scripts & deliverable for vendors to establish their capabilities in the context of your organization’s demands.
To fulfill the objective, an efficient evaluation should be drawn within adequate time and resources allocated for the project. The original request for proposal and its design has multiple functions: Keys documents provide responses to requirements; the license structure ensures cross-vendor comparisons can be made; implementation, project management, and support ensure that the design can be implemented. Evaluations of all demonstrations should be captured as best practice in a common platform for better communication. In demonstration, the buyer’s identified needs are to be fulfilled by the vendor. It is still important to allocate appropriate time for the vendor to differentiate and demonstrate specific requirements.
Organizations can select the implementation planning directed in parallel with the performance testing of software. The achievement of the milestone is based on the validation of performance and availability. The original team was trained in a train-the-trainer approach, and key personnel on the original team were equipped with the necessary skills to represent the organization. The approach taken across either overseas sites or multi-site organization was for individual plants to determine their own timeline for implementation. So, with this model, you are ready to establish and minimize interruption.
One significant point of learning for this session was the fact that the deployment is and will continue to be (just like cGMP) subject to continuous improvement. Configuration changes, reporting, and analytical approaches as well as many other sides will change with underlying processes as it will the core solution. The organization can be responsible for identifying optimistic changes in the software. Wherever required, changes submitted to the vendor for enclosure in new releases to align with current regulatory demands. The journey is one that will continue for the life of the solution Doc Control data serves automates.
Only the regulatory drug manufacturers know about the advantage of implementing document control software. Other stakeholders are not usually aware about the benefits. Over and above a user’s common interaction with the integrated solution is crucial to share success in terms of quality business benefits and advantages seen in the management of product compliance and transparency across organization. Hence, by seamlessly interfacing with the ERP, LIMS solution, Doc Control allowed robust tracking business processes for management.
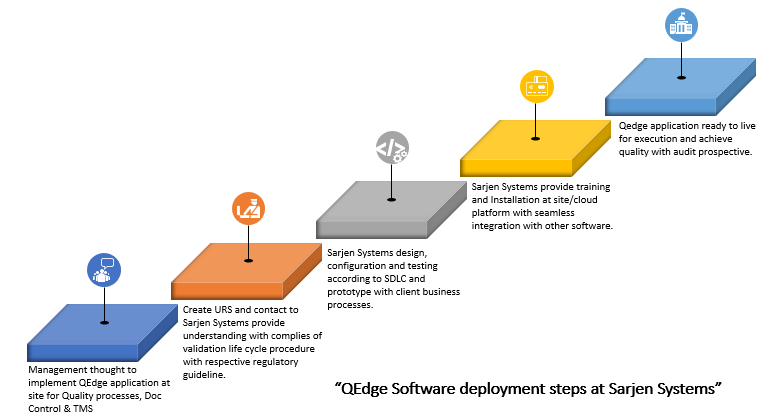
The above diagram explains our robust and powerful solution QEdge. Likewise, an enterprise wide quality management solution catering to activities like Quality Processes, Doc Control and Training management. The involved employees and organization constitute the most important resource for improving quality and data readily available for regulatory audits.